Part:BBa_K1399004:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1399004
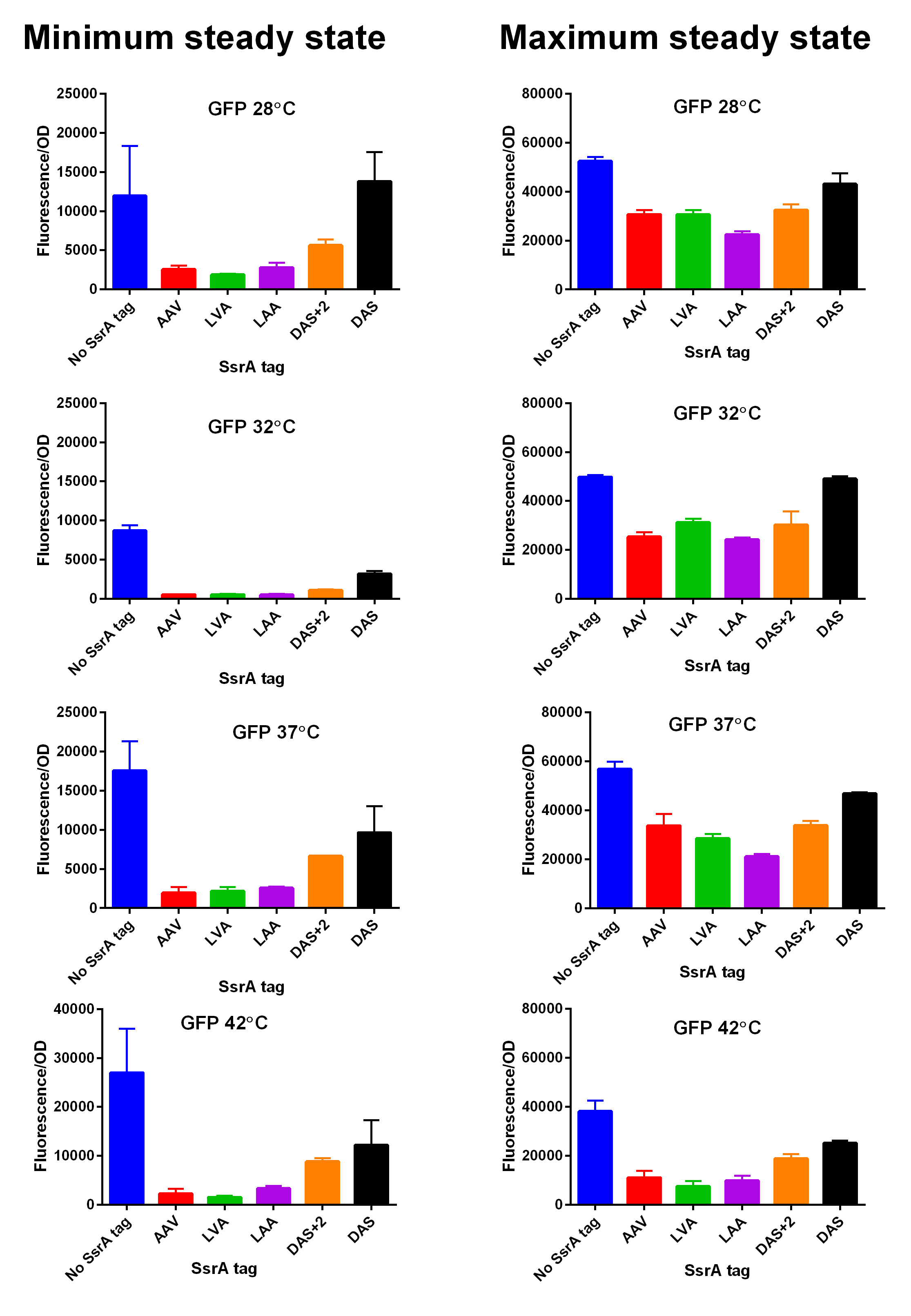
We constructed lactose/IPTG inducible measurement pathway for this part (Part:BBa_K1399015) to characterize and compare the instability of this SsrA-LVA tagged GFP to others containing different variants of SsrA tags. We determined the steady state fluorescence/OD ratio of E. coli JM109 at uninduced (minimum steady state) and induced (maximum steady state) state. To see how the temperature of environment can affect protein degradation efficiency we repeated experiments in several temperatures (28⁰C, 32⁰C, 37⁰C and 42⁰C). Our data are summarised below. (For more information on SsrA degradation tag system, our experimental assays and results please visit our wiki page [http://2014.igem.org/Team:Edinburgh].)
iGEM17_UCAS Experience
Improvement of BBa_K1399004
Considering that the highly efficient folding capability of superfolder GFP (sfGFP) should be more exploited, we replaced the mut3b GFP with a superfolder GFP, to see if there is any improvement for this part. This time, we took the part BBa_K1339004, which is a mut3b GFP with a LVA tag, as a try, and the improved part is BBa_K2287000.
By comparing the kinetic curves of these two parts (see [http://2017.igem.org/Team:UCAS/Notebook methods]), we observed that there is no remarkable improvement in total relative fluorescence intensity at the steady state, and the degradation trends are highly similar as well (fig.1). The minimum fluorescence intensity of sfGFP with the LVA tag is a little bit weaker than mut3b GFP, which may because the set wavelength of the exciting light suits the mut3b GFP best but not the sfGFP. In another word, this may not be seen as an improved feature.
However, when analyzing the growth curves, we found that the curve of mut3b GFP reveals stronger fluctuation than sfGFP. That is to say, the sfGFP with a LVA tag shows a more stable growth state (fig.2). This is a significant improvement, because for engineered bacteria expected to work properly in real world, they must have robust viability. A part leaves bacteria growing unstably cannot be further programmed efficiently.
User Reviews
UNIQ7f347e497e67eb28-partinfo-00000000-QINU
|
•••••
UCAS 2017 |
This part is a useful part in synthetic biology. It can help engineer controllable protein degradation. However, the reporter employed here can be further improved, hence we replaced it with sfGFP. |
UNIQ7f347e497e67eb28-partinfo-00000002-QINU